In production and life, silica gel can be used to dry N2, air, hydrogen, natural gas [1] and so on. According to acid and alkali, desiccant can be divided into: acid desiccant, alkaline desiccant and neutral desiccant [2]. Silica gel appears to be a neutral dryer that seems to dry NH3, HCl, SO2, etc. However, from the principle point of view, silica gel is composed of three-dimensional intermolecular dehydration of orthosilicic acid molecules, the main body is SiO2, and the surface is rich in hydroxyl groups (see Figure 1). The reason why silica gel can absorb water is that the silicon hydroxyl group on the surface of silica gel can form intermolecular hydrogen bonds with water molecules, so it can adsorb water and thus play a drying role. The color-changing silica gel contains cobalt ions, and after the adsorption water reaches saturation, the cobalt ions in the color-changing silica gel become hydrated cobalt ions, so that the blue silica gel becomes pink. After heating the pink silica gel at 200℃ for a period of time, the hydrogen bond between the silica gel and water molecules breaks, and the discolored silica gel will turn blue again, so that the structure diagram of the silicic acid and silica gel can be reused as shown in Figure 1. So, since the surface of silica gel is rich in hydroxyl groups, the surface of silica gel may also form intermolecular hydrogen bonds with NH3 and HCl, etc., and there may be no way to act as a desiccant of NH3 and HCl, and there is no relevant report in the existing literature. So what were the results? This subject has done the following experimental research.
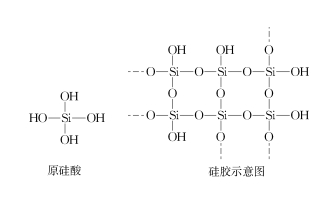
FIG. 1 Structure diagram of ortho-silicic acid and silica gel
2 Experiment Part
2.1 Exploration of the scope of application of silica gel desiccant — Ammonia First, the discolored silica gel was placed in distilled water and concentrated ammonia water respectively. Discolored silica gel turns pink in distilled water; In concentrated ammonia, the color-changing silicone first turns red and slowly turns light blue. This shows that silica gel can absorb NH3 or NH3 ·H2 O in ammonia. As shown in Figure 2, solid calcium hydroxide and ammonium chloride are evenly mixed and heated in a test tube. The resulting gas is removed by alkali lime and then by silica gel. The color of the silica gel near the entrance direction becomes lighter (the color of the application scope of the silica gel desiccant in Figure 2 is explored — ammonia 73, the 8th phase of 2023 is basically the same as the color of the silica gel soaked in concentrated ammonia water), and the pH test paper has no obvious change. This indicates that the NH3 produced has not reached the pH test paper, and it has been completely adsorbed. After a period of time, stop the heating, take out a small part of the silica gel ball, put it into the distilled water, add phenolphthalein to the water, the solution turns red, indicating that the silica gel has a strong adsorption effect on NH3, after the distilled water is deattached, NH3 enters the distilled water, the solution is alkaline. Therefore, because the silica gel has a strong adsorption for NH3, the silicone drying agent can not dry NH3.
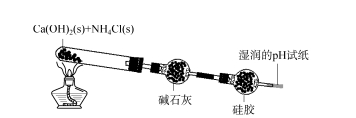
FIG. 2 Exploration of the scope of application of silica gel desiccant — ammonia
2.2 Exploration of the scope of application of silica gel desiccant — hydrogen chloride first burns NaCl solids with alcohol lamp flame to remove the wet water in the solid components. After the sample is cooled, concentrated sulfuric acid is added to NaCl solids to immediately produce a large number of bubbles. The generated gas is passed into a spherical drying tube containing silica gel, and a wet pH test paper is placed at the end of the drying tube. The silica gel at the front end turns light green, and the wet pH test paper has no obvious change (see Figure 3). This shows that the generated HCl gas is completely adsorbed by silica gel and does not escape into the air.
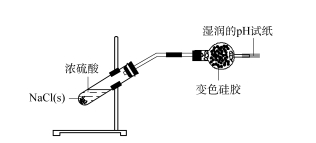
Figure 3 Research on the scope of application of silica gel desiccant — hydrogen chloride
The silica gel adsorbed HCl and turned light green was placed in a test tube. Put the new blue silica gel in the test tube, add concentrated hydrochloric acid, silica gel also becomes light green color, the two colors are basically the same. This shows the silica gel gas in the spherical drying tube.
2.3 Exploration of the application scope of silica gel desiccant — sulfur dioxide Mixed concentrated sulfuric acid with sodium thiosulfate solid (see Figure 4), NA2s2 O3 +H2 SO4 ==Na2 SO4 +SO2 ↑+S↓+H2 O; The generated gas is passed through the drying tube containing the discolored silica gel, the discolored silica gel becomes light blue-green, and the blue litmus paper at the end of the wet test paper does not change significantly, indicating that the generated SO2 gas has been completely adsorbed by the silica gel ball and cannot escape.
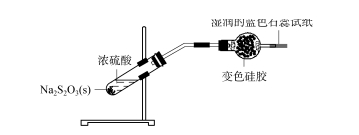
FIG. 4 Exploration of the scope of application of silica gel desiccant — sulfur dioxide
Take off a part of the silica gel ball and put it in distilled water. After full balance, take a small amount of water drop on the blue litmus paper. The test paper does not change significantly, indicating that distilled water is not enough to desorbed SO2 from the silica gel. Take a small part of the silica gel ball and heat it in the test tube. Put wet blue litmus paper at the mouth of the test tube. The blue litmus paper turns red, indicating that heating makes SO2 gas desorbed from the silica gel ball, thus making the litmus paper turn red. The above experiments show that silica gel also has a strong adsorption effect on SO2 or H2 SO3, and can not be used for drying SO2 gas.
2.4 Exploration of the scope of application of silica gel desiccant — Carbon dioxide
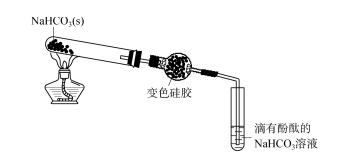
As shown in Figure 5, sodium bicarbonate solution dripping phenolphthalein appears light red. The sodium bicarbonate solid is heated and the resulting gas mixture is passed through a drying tube containing dried silica gel spheres. The silica gel does not change significantly and the sodium bicarbonate dripping with phenolphthalein adsorbs the HCl. The cobalt ion in the discolored silica gel forms a green solution with Cl- and gradually becomes colorless, indicating that there is a CO2 gas complex at the end of the spherical drying tube. The light-green silica gel is placed in distilled water, and the discolored silica gel gradually changes to yellow, indicating that the HCl adsorbed by silica gel has been desorbed into the water. A small amount of the upper aqueous solution was added to the silver nitrate solution acidified by nitric acid to form a white precipitate. A small amount of aqueous solution is dropped on a wide range of pH test paper, and the test paper turns red, indicating that the solution is acidic. The above experiments show that silica gel has a strong adsorption to HCl gas. HCl is a strongly polar molecule, and the hydroxyl group on the surface of silica gel also has strong polarity, and the two may form intermolecular hydrogen bonds or have relatively strong dipole dipole interaction, resulting in a relatively strong intermolecular force between the surface of silica gel and HCl molecules, so silica gel has a strong adsorption of HCl. Therefore, silicone drying agent can not be used to dry HCl escape, that is, the silica gel does not adsorb CO2 or only partially adsorb CO2.
FIG. 5 Exploration of the scope of application of silica gel desiccant — carbon dioxide
In order to prove the adsorption of silica gel to carbon dioxide gas, the following experiments are continued. The silica gel ball in the spherical drying tube was removed, and the part was divided into sodium bicarbonate solution dripping phenolphthalein. The sodium bicarbonate solution was decolored. This shows that silica gel adsorbs carbon dioxide, and after soluble in water, carbon dioxide desorbs into sodium bicarbonate solution, making sodium bicarbonate solution fade. The remaining part of the silicone ball is heated in a dry test tube, and the resulting gas is passed into a solution of sodium bicarbonate dripping with phenolphthalein. Soon, the sodium bicarbonate solution changes from light red to colorless. This also shows that silica gel still has adsorption capacity for CO2 gas. However, the adsorption force of silica gel on CO2 is much smaller than that of HCl, NH3 and SO2, and carbon dioxide can only be partially adsorbed during the experiment in Figure 5. The reason why silica gel can partially adsorb CO2 is likely to be that silica gel and CO2 form intermolecular hydrogen bonds Si — OH… O =C. Because the central carbon atom of CO2 is sp hybrid, and the silicon atom in silica gel is sp3 hybrid, the linear CO2 molecule does not cooperate well with the surface of silica gel, resulting in the adsorption force of silica gel on carbon dioxide is relatively small.
3.Comparison between the solubility of the four gases in water and the adsorption status on the surface of silica gel From the above experimental results, it can be seen that silica gel has a strong adsorption capacity for ammonia, hydrogen chloride and sulfur dioxide, but a small adsorption force for carbon dioxide (see Table 1). This is similar to the solubility of the four gases in water. This may be because water molecules contain hydroxy-OH, and the surface of silica gel is also rich in hydroxyl, so the solubility of these four gases in water is very similar to its adsorption on the surface of silica gel. Among the three gases of ammonia gas, hydrogen chloride and sulfur dioxide, sulfur dioxide has the smallest solubility in water, but after being adsorbed by silica gel, it is the most difficult to desorption among the three gases. After the silica gel adsorbs ammonia and hydrogen chloride, it can be desorbed with solvent water. After the sulfur dioxide gas is adsorbed by silica gel, it is difficult to desorption with water, and must be heated to desorption from the surface of silica gel. Therefore, the adsorption of four gases on the surface of silica gel must be theoretically calculated.
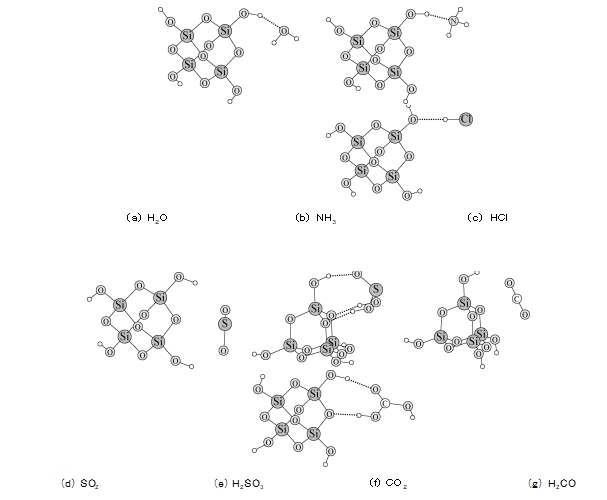
4 Theoretical calculation of the interaction between silica gel and four gases is presented in the quantumization ORCA software [4] under the framework of density functional theory (DFT). The DFT D/B3LYP/Def2 TZVP method was used to calculate the interaction modes and energies between different gases and silica gel. In order to simplify the calculation, silica gel solids are represented by tetrameric orthosilicic acid molecules. The calculation results show that H2 O, NH3 and HCl can all form hydrogen bonds with the hydroxyl group on the surface of silica gel (see Figure 6a ~ c). They have relatively strong binding energy on the silica gel surface (see Table 2) and are easily adsorbed on the silica gel surface. Since the binding energy of NH3 and HCl is similar to that of H2 O, water washing can lead to desorption of these two gas molecules. For the SO2 molecule, its binding energy is only -17.47 kJ/mol, which is much smaller than the above three molecules. However, the experiment confirmed that SO2 gas is easily adsorbed on the silica gel, and even washing can not desorbed it, and only heating can make SO2 escape from the surface of the silica gel. Therefore, we guessed that SO2 is likely to combine with H2 O on the surface of silica gel to form H2 SO3 fractions. Figure 6e shows that the H2 SO3 molecule forms three hydrogen bonds with the hydroxyl and oxygen atoms on the surface of the silica gel at the same time, and the binding energy is as high as -76.63 kJ/mol, which explains why SO2 adsorbed on the silica gel is difficult to elude with water. Non-polar CO2 has the weakest binding ability with silica gel, and can only be partially adsorbed by silica gel. Although the binding energy of H2 CO3 and silica gel also reached -65.65 kJ/mol, the conversion rate of CO2 to H2 CO3 was not high, so the adsorption rate of CO2 was also reduced. It can be seen from the above data that the polarity of the gas molecule is not the only criterion to judge whether it can be adsorbed by silica gel, and the hydrogen bond formed with the silica gel surface is the main reason for its stable adsorption.
The composition of silica gel is SiO2 ·nH2 O, the huge surface area of silica gel and the rich hydroxyl group on the surface make silica gel can be used as a non-toxic dryer with excellent performance, and is widely used in production and life. In this paper, it is confirmed from two aspects of experiment and theoretical calculation that silica gel can adsorb NH3, HCl, SO2, CO2 and other gases through intermolecular hydrogen bonds, so silica gel can not be used for drying these gases. The composition of silica gel is SiO2 ·nH2 O, the huge surface area of silica gel and the rich hydroxyl group on the surface make silica gel can be used as a non-toxic dryer with excellent performance, and is widely used in production and life. In this paper, it is confirmed from two aspects of experiment and theoretical calculation that silica gel can adsorb NH3, HCl, SO2, CO2 and other gases through intermolecular hydrogen bonds, so silica gel can not be used for drying these gases.
3
FIG. 6 Interaction modes between different molecules and silica gel surface calculated by DFT method
Post time: Nov-14-2023